About Deuterium
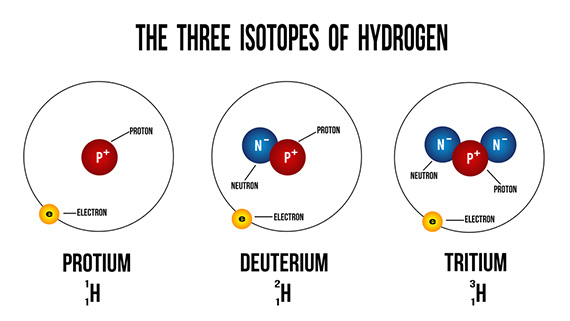
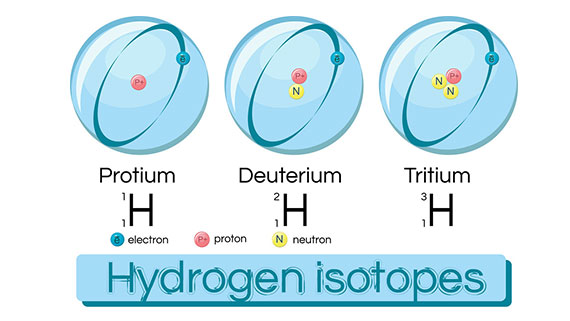
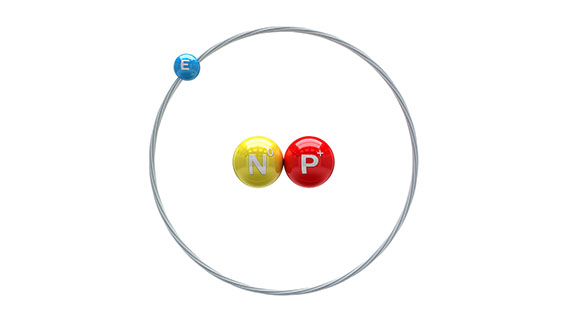
Deuterium (symbol: Hydrogen-2, D or 2H), also known as heavy Hydrogen. is one of the two stable isotopes of hydrogen. The other one is Protium (symbol: Hydrogen-1, 1H). The nucleus of Deuterium, called Deuteron, contains one proton and one neutron, whereas the far more common, Protium has no neutron in the nucleus. Deuterium has a natural abundance in Earth’s oceans of about 1 atom to 6420 atoms of hydrogen. Thus deuterium accounts for approximately 0.0156% (or 0.0312%, on a mass basis) of all the naturally occurring hydrogen in the oceans, while Protium accounts for more than 99.98%. The abundance of deuterium changes slightly from one kind of natural water to another (see Vienna Standard Mean Ocean Water).
What is deuterium?
Deuterium is one of the two hydrogen isotopes. The nucleus of most hydrogen atoms in nature contains one proton…
Deuterium in nature
The deuterium content of waters in our climate area is 150 ppm (parts per million), with a minimal fluctuation…
Deuterium properties
Deuterium can have kinetic isotopic effects different than hydrogen has, and the physical and chemical properties of…